Greenlight Guru Announces Advanced Document Management

Latest News
September 5, 2018
Greenlight Guru, quality management software designed specifically for medical device companies, now offers Advanced Document Management, the latest version of its purpose-built software.
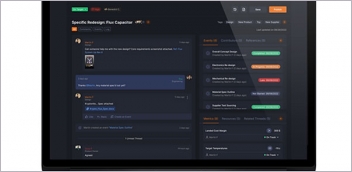
Advanced Document Management enables collaborative sharing among teams while maintaining security levels through use of its permissions feature. These granular controls give teams from small, midsize and enterprise organizations flexibility in assuring the right people have access to the right documents in real time, the company reports. Advanced Document Management is built to scale, allowing documents to be shared with individuals, roles or geographic teams. The latest release also includes an enhanced user interface for more dynamic and intuitive document lists, including filtering by document classification or workflow routing statuses.
“We set out to make the lives of patients better as well as the lives of those designing the next generation of medical devices,” says Greenlight Guru co-founder and CEO David DeRam. “Keeping documents, records and procedures secure, up-to-date and always accessible in a spreadsheet or paper-based system is a never-ending task that often leads to mistakes and the inevitable question, ‘which version of the document is most up-to-date?’ Advanced Document Management ensures documents are secured and access is properly controlled across the entire organization in minutes, not hours.”
The medical device industry surpassed more than $370 billion in salesin 2017; however, concurrent to this growth is the rise in adverse events resulting in patient death and injuries, as well as product recalls.
The U.S. Food and Drug Administration is partnering with medical device industry stakeholders—including Greenlight Guru—to develop a focus on quality rather than compliance only. Tools such as Advanced Document Management can reportedly reduce the administrative load associated with maintaining compliance and allow device makers to focus on developing high quality devices, the company reports.
“Errors in document management is a common source of delay in device reviews and approvals,” says Greenlight Guru founder and VP of QA/RA Jon Speer. “This update will allow product managers to assign documents to the relevant teams – and no one else—hereby keeping workflows free of documents that aren’t relevant to that team’s function. Additionally, our new document sorting and filtering capabilities will reduce the time needed to locate and produce documents for approvals and audits, allowing manufacturers to focus less on paperwork and more on creating quality medical devices.”
This product update follows Greenlight Guru’s Multi-Level Design Controls update and partnership with the FDA to provide meaningful insight into the FDA’s Case for Quality Initiative.
For more info, visit Greenlight Guru.
Sources: Press materials received from the company.
Subscribe to our FREE magazine, FREE email newsletters or both!
Latest News
About the Author
DE’s editors contribute news and new product announcements to Digital Engineering.
Press releases may be sent to them via [email protected].