Latest News
April 1, 2013
By Cheryl Liu
| A |  |
| B | 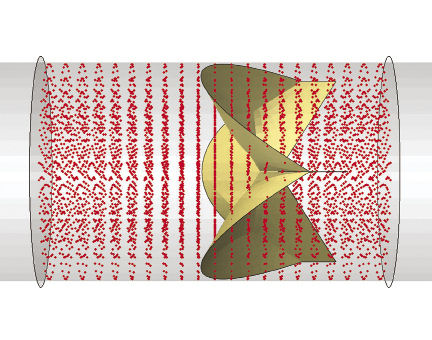 |
| C | 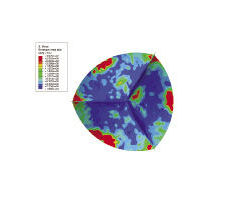 |
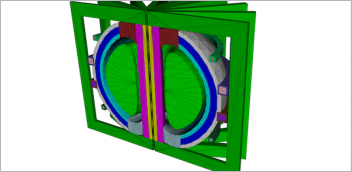
| Figure 1: This example of modeling and simulation of a medical device shows an aortic valve geometry (A), a model of the effect of blood flow on the valve in a blood vessel (B), and an Abaqus FEA of the stress on the valve leaflets during the diastolic phase (C). This work was performed by SIMULIA, Dassault Systemes, in conjunction with the FDA’s Center for Devices and Radiological Health. | |
One of the toughest design engineering challenges is making a medical device that works flawlessly with the human body. The unique anatomy and physiology of every patient create physical complexities—and ever-shifting functional parameters, that must be thoroughly accounted for when producing a therapeutic product that may need to last a lifetime.
Domestic inpatient procedures involving medical devices—stents, heart valves, dental implants, spine and joint implants, surgical tools, blood pump, endovascular grafts, drug-eluting devices and more—totaled 46 million in the U.S. alone in 2006, according to the U.S. Centers for Disease Control (CDC). It’s a global market that is growing along with aging populations everywhere.
Medical device companies and their designers are increasingly viewing computer simulation, already widely accepted in many industries, as an important tool. It helps them visualize what they cannot see, explore the design space more fully, refine their ideas faster and more accurately—and reduce expensive prototyping and testing.
Solid mechanics simulations can help determine proper implant size, evaluate manufacturing tolerances, compare design geometries or consider next-generation devices. Computational fluid dynamics (CFD) can be employed to identify high-shear stresses on blood vessels, regions of low flow, and potential for blood damage. And simulation-based product development processes can be linked in automated workflows—optimizing huge quantities of design data to provide fine-tuned results that are of particular value for creating patient-specific medical devices.
As life sciences engineers embrace simulation, they are achieving increasingly accurate levels of precision when evaluating device function, including the ability to evaluate aspects of device performance not possible with bench tests alone. As a result, the U.S. Food and Drug Administration’s (FDA’s) Center for Device and Radiological Health (CDRH) is seeing a growing number of submissions for medical devices that include a simulation-data component.
The CDRH is responsible for regulating firms that manufacture, repackage, re-label and/or import medical devices sold in the United States. The submissions for these therapeutic devices typically contain data from four types of evaluation models—animal, bench, computational and human—to demonstrate a reasonable assurance of safety and effectiveness.
When a company submits simulation metrics that supplement bench testing, this can help promote approval by demonstrating both the integrity of the proposed device and the required realistic device failure analysis. As the ultimate safety-and-effectiveness regulatory body between medical device manufacturers and patients, the FDA recognizes the value of such advancing technologies—and its own need to stay abreast of them. The agency has now begun actively encouraging the use of simulation in device evaluation.
However, the FDA has also put the industry on notice that verification and validation must go hand-in-hand with the use of simulation in applications. The CDRH is looking to quantify when a computational model is credible enough, and that its intended purpose is appropriate for a regulatory submission. Unclear reporting standards, insufficient data about geometries and boundary conditions, lack of validation metrics, incomplete understanding of physiological loads in the body, and variations in patient populations—any and all of these uncertainties can impact the relevance of simulation outputs.
SIMULIA Contributes to Knowledge Advancement
Noticing that a significant proportion of the applications the agency has seen in recent years have included simulations with Abaqus finite element analysis (FEA) from SIMULIA, Dassault Systemes, the CDRH reached out to SIMULIA in 2010 for support in developing its own internal framework, and in-house expertise, for validating and regulating industry-submitted simulations.
SIMULIA presented at the FDA’s third workshop on Computational Modeling of Medical Devices the same year. It continues to deliver on-site training courses to FDA reviewers about best practices in modeling and simulation, and to partner with the FDA on aortic valve model development (see Fig. 1). The FDA has also presented at the SIMULIA Community Conference and Regional User Meetings.
 Figure 2: Broad cross-industry collaboration among medical device manufacturers, academia and software companies is being harnessed for the FDA’s Virtual Physiological Patient project. |
Realizing the importance of model verification and validation (V&V), in early 2011, ASME and FDA launched the V&V 40 subcommittee to develop V&V guidelines for the medical device industry specifically; SIMULIA is actively participating, along with others in the industry and software communities.
As one outcome of these efforts, this year the FDA will publish a guidance document titled “Reporting Computational Modeling Studies in Medical Device Regulatory Submissions.” Appendices will cover fluid and mass transport, solid mechanics, electromagnetism, control loops, thermal transport, and ultrasound. Publication date updates can be found on the CDRH website.
The ‘Virtual Patient’ Idea is Born
As knowledge about the importance of simulation grows, another priority for the FDA is the creation of a publicly available “Virtual Physiological Patient” of human body computer models in different disease states (see Fig. 2). This is not intended to be a single model encompassing every function and disease at once. Rather, the project will comprise a library of verified and validated submodels and data based on the combined expertise of those groups in the relevant disciplines—cardiology, orthopedics, software and so forth.
| MDIC Partnership Benefits All Concurrent with the development of the Virtual Physiological Patient concept, the U.S. Food & Drug Administration (FDA) is reaching outward to device manufacturers, software providers and medical professionals to form a Regulatory Science Public-Private Partnership. Launched in December 2012, the partnership is called the Medical Device Innovation Consortium (MDIC). Specific details are available at DeviceConsortium.org. The idea is to create an opportunity for information gathering in a pre-competitive state—that is, not device-specific, but disease-specific. For example, if the heart valve community were interested in a comprehensive evaluation of the structure and function of heart valves, costs could be minimized through non-profit group funding and participation in the development of tools and resources for modeling and simulating of a range of valves. All results would be shared. End-stage renal disease is another area recently identified by the FDA as a priority. Industry forums on this topic are already under way. The medical device industry can only benefit from such endeavors. Individual device design copyrights certainly need to be protected, but the tradition of publishing evidence-based research results to move the entire body of medical knowledge forward has resonated in the life sciences throughout the history of medicine. A deep understanding of the function of the living body is critical to every medical-device developer, and sharing the data that lie at the core of that understanding can be accomplished without infringing on any one company’s patents. —C. Liu |
The goal of the Virtual Physiological Patient project is a shared point of reference that will improve understanding of model attributes and limitations, and provide discrete models, data and simulations validated for regulatory evaluation. Peer review by experts in academic, government and industry will ensure robust V&V, and provide periodic assessment. SIMULIA is contributing expertise to a group that is developing a computational model for the evaluation of a diseased femoral artery for stent evaluation.
| Surgery Simulator Cleared In addition to approving the use of simulation in medical device evaluations, the U.S. Food & Drug Administration (FDA) also recently cleared Surgical Theater, LLC’s Selman Surgery Rehearsal Platform (SRP), making it the only patented and FDA cleared platform for cerebral and spine pre-surgery rehearsal, according to the company. Using standard scanned images from any patient, the SRP generates 3D patient-specific models showing the interaction between life-like tissue and surgical instruments. The tissue responds “realistically” to actions taken by the surgeon, enabling accurate pre-surgery planning and rehearsal. The software uses flight simulator technology to permit the remote connection of multiple SRPs so participants anywhere in the world can simultaneously work together and practice the same case with real-time feedback and collaborate on the planning of a specific surgery case. For more information, visit surgicaltheater.net. —DE staff |
The FDA views modeling and simulation as incentives to innovation that can reduce the time and cost of device design, assessment and manufacturing. It is the best interests of the medical device industry, the regulatory agency and software companies to collaborate to ensure that the power of simulation is increasingly utilized to solve the wide range of challenges in medical device development. The ultimate goal is the safety and effectiveness of medical devices for every physician who uses them, and every patient who needs them.
Author’s Note: Special thanks to Drs. Tina Morrison, Nandini Duraiswamy and Donna Lochner of the FDA for their assistance in preparing this article.
Cheryl Liu is life sciences senior technical lead, SIMULIA, Dassault Systemes. Send e-mail about this article to [email protected].
More Info
U.S. Food & Drug Administration
Read more about how the FDA is promoting innovation in “High Stakes Balancing Act” in Dassault Systemes’ COMPASS magazine: CompassMag.3ds.com
Subscribe to our FREE magazine, FREE email newsletters or both!
Latest News